Introduction:
Suppositories are solid
dosage forms of various sizes, appearance (shapes) and weights intended for
administration commonly by rectal route, vaginal route or even urethral where
they melt, soften or dissolve to exert their effect. Suppositories come in
variety of sizes and shapes where they are capable of being easily inserted
into the intended orifice without causing undue distention. Moreover,
suppositories can be used for two main purposes. The first is to act locally as
a protective around the tissue where it is inserted and on the other hand
suppository can not only act simply as a vehicle or carrier of an active drug,
that we want to be absorbed at site of action but it can also be distributed
systematically to produce an effect throughout the entire body.
The suppository usually
composed of a medicament incorporated (dissolved or suspended) in a suppository
base, this medicament may be intended for retention within the cavity for
localized drug effect or to be absorbed for the exertion of systemic effect.
For example, rectal localized action such as relief of constipation, pain,
itching and inflammation associated with hemorrhoid conditions. Suppositories
are indicated for systemic action in pediatric patients and in patients who
cannot take or tolerate oral medication due to variety of reasons e.g. to
relief nausea, vomiting and pain. The drug must be spread in a suitable base of
suppository. Ideal suppository bases should be easily formed by compression or
molding; release any medicament readily; melt at body temperature or dissolve
or disperse in body fluids; keep its shape when handled; compatible with the
drugs, non-irritant and non-toxic.
Polyethylene glycol
(PEG) polymers have received much attention as suppository bases in recent
years because they possess many desirable properties. They are chemically
stable, non-irritating, miscible with water and mucous secretions, and can be
formulated, either by mold or compression, in a wide range of hardness and
melting point. Moreover, they do not melt at body temperature, but dissolve to
provide a prolonged release. Certain PEG polymers may be used singly as
suppository bases but, more commonly, formulas call for compounds of two or
more molecular weights mixed in various proportions as needed to yield a
finished product of satisfactory hardness and dissolution time.
Objectives:
·
To calibrate
suppository mould with PEG before preparing medicated suppositories.
·
To determine the
effect of different compositions of PEG base on the physical characteristics of
suppositories.
3.0 Materials and
methodology:
3.1 Apparatus:
|
·
Water bath at 37oC
|
·
1 x Suppository mould set
|
|
·
Hotplate
|
·
1 x Spatula
|
|
·
4 x 50 mL beaker
|
·
4 x Weighing boats
|
|
·
1 x 5 mL pipette and pipette
bulb
|
·
2 x Glass rod
|
|
·
1 x 5 mL measuring cylinder
|
·
Analytical balance
|
3.2 Materials:
|
·
Polyethylene glycol (PEG) 1000
|
·
Distilled water
|
|
·
Polyethylene glycol (PEG) 6000
|
·
Liquid paraffin
|
|
·
Paracetamol
|
|
3.3 Methodology
3.3.1 Calibration of
Suppository Molds with PEG Base
For the calibration
exercise, 10 g of following proportion PEG 1000 and PEG 6000 had been used.
|
Ingredients
|
Percentage
|
Weight Basis
|
|
PEG 1000
|
60%
|
6.00 g
|
|
PEG 6000
|
40%
|
4.00 g
|
To calibrate the mold
with PEG suppository base:
1. A clean and dry mold
is taken and the mold is not lubricated.
2. The PEG 1000 is
melted on a steam bath or hot plate, then, the heat is reduced and mixed with
other PEG.
3. The mixture is
removed from the heat and is allowed to cool before pouring into the mold.
4. The cavities are
overfilled in the mold and is left to solidify at room temperature.
5. The excess is
carefully removed with a hot spatula; the suppositories were removed from the
mold.
6. The suppositories
are weighed and the total weight is recorded. The average suppository weight is
calculated.
3.3.2 Preparation of
paracetamol suppositories
1. Saturated stock
solution of paracetamol is prepared by adding 10 g of paracetamol in 5 mL
distilled water.
2. Paracetamol
suppository is prepared using the following formulation:
|
Suppository
|
PEG 1000
(g)
|
PEG 6000
(g)
|
Paracetamol
stock solution (mL)
|
Total
(g)
|
|
I
|
9
|
0
|
1
|
10
|
|
II
|
6
|
3
|
1
|
10
|
|
III
|
0
|
9
|
1
|
10
|
3. Each type of PEG is
melted on the steam bath or hot plate, then, the heat is reduced and mixed with
other PEG.
4. The mixture is
removed from heat and is allowed to cool down before pouring into the mold.
5. The cavities are overfilled in the mold and is
left to solidify at room temperature.
6. The excess is
carefully removed with a hot spatula; the suppositories were removed from the
mold.
7. The shape, texture
and colour of the suppositories are observed.
8. Each of the suppositories are put into separate
beaker containing distilled water ( 10 mL and pre-warmed at 37 oC) and the beaker is put into the water bath.
9. The time for
the suppositories to melt is recorded.
Results:
Calibration of suppository molds with PEG base.
|
Mold
|
|
|
Total
weight for 6 suppositories
|
6.457
g
|
|
Average
weight for 1 suppository
|
1. 076g
|
|
Suppositories
|
I
|
II
|
III
|
|
Time taken to melt
(min)
|
47.34
|
42.11
|
54.48
|
|
Shape
|
Bullet
|
Bullet
|
Bullet
|
|
Hardness
|
Low
|
Medium
|
High
|
|
Greasiness
|
High
|
Medium
|
Low
|
|
Color
|
Clear White
|
Clear White
|
Pale White
|
Discussion :
1. Describe the important of
calibrating suppository mould before preparing medicated suppository
The
calibration of suppository mould is required to be done as each individual mold
is capable of holding a specific volume of material in each of its openings.
For example, the weight of suppository made up of cocoa butter will differ from
the weight of suppository prepared in the same mold with a polyethylene glycol
base (PEG) due to the differences in the density of the base materials.
Therefore, any addition of medicinal agents alters the density of the base and
the weight of the resulting suppository will differ from the suppository that
has been prepared with base material alone. This is the reason why a
pharmacists calibrates the suppository mold with a common base; to ensure that
the medicated suppository will contain proper amount of medicament.
2. Compare the physical appearance of suppositories that are formed and
discuss.
The shape for all Paracetamol
suppositories are bullet shape because they are made using the same mold. The
higher the molecular weight, the more hydroxyl group, the higher its melting
point. As a result, the melting point for suppository III, which has 9g of PEG
6000,
is the longest and supposedly the melting point point for suppository I is the
fastest but somehow our result deviate because suppository II melt faster. This could be due to error while conducting
this experiment. The same relationship
can be used to explain the hardness of the suppositories, the more amount of
PEG 6000 the harder
the suppository will be.. Suppository I is more greasy than the
other suppositories because it contain 9g of PEG 1000. PEG 1000 is more hydrophobic than PEG 6000 so, the higher
amount of PEG 1000, the more greasy the suppository will be.
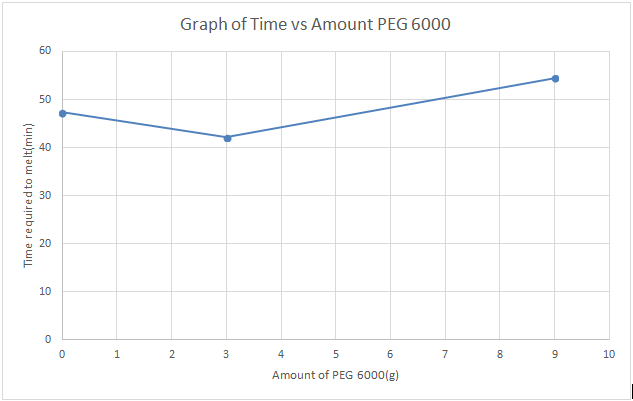
3.
From the graph,it is known that suppositories that have
higher amount of PEG 6000 melt less rapidly than others suppositories using
lesser amount of PEG 6000.This can be attributed to the physical properties of
the suppositories itself, suppositories having high amount of PEG 6000 exhibit
increase hardness and brittle properties,which upon contact with water or
aqueous solution,disintegrate more easily than those suppositories having lower
amount of PEG 6000 and appear soft.
4. Describe function(s) of each ingredients used
in the suppository formulation.
The ingredients used in the suppository formulation is
PEG 1000, PEG 6000 and paracetamol. Paracetamol is the active ingredient in the
suppository. Paracetamol is also known as acetaminophen. It has analgesic
effect and antipyretic effect. It can help to relieve pain and fever. It is
typically used for mild to moderate pain.
Polyethylene glycols are polymers of ethylene oxide and
water which has various chain lengths, molecular weights, and physical states.
They are chemically stable, nonirritating, and miscible with water and mucous
secretions and can be formulated, either by molding or compression, in a wide
range of hardness and melting point. The higher the molecular weight of PEG,
the harder the suppositories formed and the higher the melting point.. Certain
polyethylene glycol polymers may be used singly as suppository bases but, more
commonly, two or more PEG with different molecular weights are used to yield a
finished product of satisfactory hardness and dissolution time.
Since
PEG is a water miscible bases, the suppository will dissolve in body fluids and
thus, need not to be formulated to melt at body temperature. Hence, it can be
formulated with much higher melting points and thus may be safely stored at
room temperature. Formulation with higher amount of PEG 6000 compare to PEG
1000 will take a longer time to melt completely and thus, has slower release
rate of drug.
Conclusion
As a conclusion, different composition in the
suppository will affect the physical property and the rate of drug release from
the suppository. Higher amount of PEG 6000, the more harder the suppository and
the higher its melting point.