TITLE :
Characterisation of Emulsion Formulations
OBEJCTIVES :
To determine:
1. The effects of HLB surfactant on the stability of
the emulsion.
2. The effects of different oil phases used in the
formulation on the physical characteristics
and stability of the emulsion.
INTRODUCTION :
Emulsion can be defined as a
disperse system consisting two immiscible liquids where one is dispersed as
droplets in another. The liquid droplets are also known as disperse or internal
phase, while the liquid in which they are dispersed is called continuous or
external phase. The disperse phase or continuous phase of pharmaceutical emulsion are usually water and
oil.
Emulsions are thermodynamically
unstable system due to high surface tension between two phases. Generally,
emulsion can be categorised as oil-in-water emulsion (o/w) and water-in-oil
emulsion (w/o). It is stabilised by adding emulsifying agents. Hydrophile - lipophile
balance or HLB method is used to determine the quantity and type of surfactant
that is needed to prepare stable emulsion. The number 1 to 20 is assigned to
each surfactant to express numerically the size and strength of the polar
portion relative to the non-polar portion of the molecule. Usually combinations
of two emulsifying agents are used to form a more stable emulsion. HLB value
for a combination of emulsifying agents can be determined by using the
following formula:
Apparatus and materials
a. Apparatus
1.
8 test tubes 9.
1 set of 5ml pipette and bulb
2.
A 50 ml
measuring cylinder 10.
1 5ml beaker
3.
2 sets of
pasture pipettes and droppers 11. A
15ml centrifugation tube
4.
Vortex mixer 12.
Centrifugation apparatus
5.
Weighing
boat 13.
Viscometer
6.
1 set of mortar
and pestle 14.
Water bath (45oC)
7.
Light microscope 15. Refrigerator
8.
Microscope
slides
b. Materials
1.
Palm oil
2.
Arachis oil
3.
Olive oil
4.
Mineral oil
5.
Distilled water
6.
Span 20
7.
Tween 80
8.
Sudan III
solution
PROCEDURES :
1.
Each test tube labelled
and 1cm from the base of the test tube is marked.
2.
4ml of oil (according to table 1) and 4ml of
distilled water is mixed into the test tube.
(Table
1)
Group
|
Oil
|
1, 5
|
Palm
oil
|
2, 6
|
Arachis
oil
|
3, 7
|
Olive
oil
|
4, 8
|
Mineral
oil
|
3.
Span 20 and
Tween 80 is added into the mixture of oil and water (refer to table 2). The
test tube is closed and its content is mixed with vortex mixer for 45 seconds.
The time needed for the interface is recorded for the interface to reach 1cm.
The HLB value is determined for each sample. The steps 1 to 3 is repeated to
obtain an average HLB value of a duplicate.
(Table 2)
Tube no.
|
1
|
2
|
3
|
4
|
5
|
6
|
7
|
8
|
Span 20
(drops)
|
15
|
12
|
12
|
6
|
6
|
3
|
0
|
0
|
Tween 80
(drops)
|
3
|
6
|
9
|
9
|
15
|
18
|
15
|
0
|
4.
A few drops of
Sudan III solution is added to (1g) emulsion formed in a weighing boat and is
mixed homogenously. The spread of the colour in the sample is compared. Some of
the sample is spread on the glass slide and observed under the light
microscope. The appearance and globule size formed is drawn and described.
5.
A Mineral Oil
Emulsion (50ml) is prepared from the formulation below using wet gum method
according to table 3a & 3b.
(Table 3a)
Mineral oil
|
(Refer to table 3b)
|
Acacia
|
6.25 g
|
Syrup
|
5 ml
|
Alcohol
|
3 ml
|
Vanillin
|
2 g
|
Distilled water qs
|
50 ml
|
(Table 3b)
Emulsion
|
Group
|
Mineral oil (ml)
|
I
|
1, 5
|
20
|
II
|
2, 6
|
25
|
III
|
3, 7
|
30
|
IV
|
4, 8
|
35
|
6.
40g of emulsion
is placed into a 50 ml beaker and is homogenized for two minutes using a vortex
mixer.
7.
2g of emulsion
is taken (before and after homogenization) and is placed into a weighing boat
and labelled.
8.
The viscosity of
the emulsion formed after homogenization (15g in 50ml beaker) is determined
using a viscometer that is calibrated with ‘Spindle’ type LV-4. The sample is
exposed to 45oC (water bath) for 15 minutes and then to 4oC
(refrigerator) for another 15 minutes. After the exposure to the temperature
cycle is finished and the emulsion had reached room temperature (10-15minutes),
the viscosity of the emulsion is determined. Step 8 is repeated and an average
value is obtained.
9.
5g of homogenised emulsion is placed into a centrifugation tube and
centrifuged (4500 rpm, 10 minutes, 25oC). The height of separation
formed is measured and the ratio of height separation is determined.
RESULT :
VISCOSITY
Group 1/5
Readings
|
Viscosity (cP)
|
Average
|
|||||
1
|
2
|
3
|
4
|
5
|
6
|
||
Before Temperature cycle
|
42
|
42
|
36
|
24
|
30
|
24
|
33
|
After temperature cycle
|
48
|
45
|
40
|
30
|
38
|
36
|
39.5
|
Difference (%)
|
19.70%
|
||||||
Group 2/6
Readings
|
Viscosity (cP)
|
Average
|
|||||
1
|
2
|
3
|
4
|
5
|
6
|
||
Before Temperature cycle
|
276
|
288
|
264
|
294
|
342
|
290
|
292.3
|
After temperature cycle
|
318
|
330
|
330
|
306
|
312
|
314
|
318.3
|
Difference (%)
|
8.90%
|
||||||
Group 3/7
Readings
|
Viscosity (cP)
|
Average
|
|||||
1
|
2
|
3
|
4
|
5
|
6
|
||
Before Temperature cycle
|
12
|
18
|
12
|
18
|
12
|
18
|
15
|
After temperature cycle
|
54
|
42
|
42
|
42
|
42
|
36
|
43
|
Difference (%)
|
186.70%
|
||||||
Group 4/8
Readings
|
Viscosity (cP)
|
Average
|
|||||
1
|
2
|
3
|
4
|
5
|
6
|
||
Before Temperature cycle
|
3.6
|
3.9
|
6.9
|
3.9
|
18.3
|
17.1
|
9.0
|
After temperature cycle
|
14.4
|
19.2
|
10.8
|
8.4
|
7.2
|
8.4
|
11.4
|
Difference (%)
|
26.70%
|
||||||
Average Viscosity (cP)
|
Amount of mineral oil (ml)
|
|||
20
|
25
|
30
|
35
|
|
Before temperature cycle
|
33
|
292.3
|
15
|
9.0
|
After temperature cycle
|
39.5
|
318.3
|
43
|
11.4
|
Differences
|
19.70%
|
8.90%
|
186.7%
|
26.7%
|
Mineral Oil(ml)
|
Height of separation
formed(cm)
|
Average(x)
|
Ratio of
separation(y)
|
||
20
|
4.0
|
3.5
|
4.0
|
3.83
|
0.77
|
25
|
3.0
|
3.50
|
3.30
|
3.27
|
0.66
|
30
|
3.3
|
3.3
|
3.2
|
3.3
|
3.3/4.7=0.70
|
35
|
0.45
|
0.45
|
0.43
|
0.43
|
0.43
|
DISCUSSION :
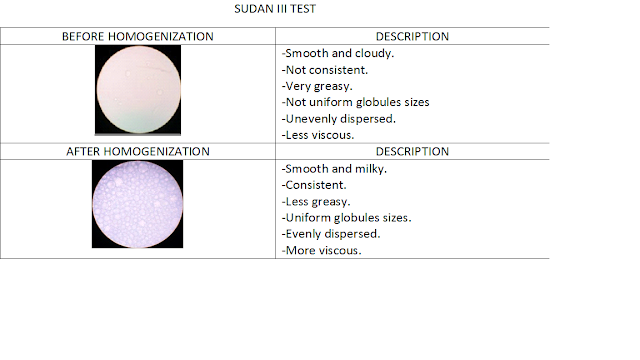
PHYSICAL APPERANCES OF OLIVE OIL EMULSIONS AND COLOUR DISPERSION PRODUCED BY SUDAN III AND SUDAN III TEST
Based on the experiment conducted by using olive oil, it happens that before homogenization the emulsion is unstable because the oily phase is immiscible with aqueous phase. The globules appear in different sizes and more large through microscope. They are unevenly being dispersed.
But after homogenization, the emulsion had become more stable as it has become less oily and has more viscosity. The globules are much smaller and evenly distributed.
VISCOSITY
Oil
is more viscous than water. Hence, theoretically, when the amount of mineral
oil increases, the viscosity of the emulsion will increases. From the
experiment, before the temperature cycle, we can see that the viscosity of
emulsions fluctuate when the amount of mineral oil increase. The emulsion with
25ml of mineral oil has the highest viscosity which is 292.3 cP while the one
with 35ml of mineral oil has the lowest viscosity which is 9.0 cP. This may be
due to some error during the experiment and the use of different viscometer.
Theoretically, the viscosity should have increased from the emulsion with
lowest amount of mineral oil to the highest.
When
the temperature increases, the viscosity of emulsion decreases. The oil
globules gain more kinetic energy and can collide with each other more
frequently. This may results in coalescence and breaking of the emulsion. While
at low temperature, the kinetic energy of oil globules reduces. The viscosity
of the emulsion will increase and it is harder for the oil globule to collide
with each other and reduces the chances for coalescence. Hence, emulsion is
more stable at low temperature. Thus, after the temperature cycle, the
viscosity of the emulsion increases. Theoretically, the difference of viscosity is directly proportional to the amount of
oil. Due to the error in the experiment, all four emulsions showed increase in
the viscosity after temperature cycle but the increase is not proportional to
the amount of mineral oil.
CONCLUSION :
1)
Different types of suspension will have
different viscosity.
2)
Based on this experiment, it can be concluded
that by using olive oil, emulsion can be stabilized after homogenization. It is
less oily and more viscosity.
3)
The more viscous, the emulsion will be more
stable.
4)
Hypothesis made is accepted.